Organic Compounds Containing Halogens Question 179
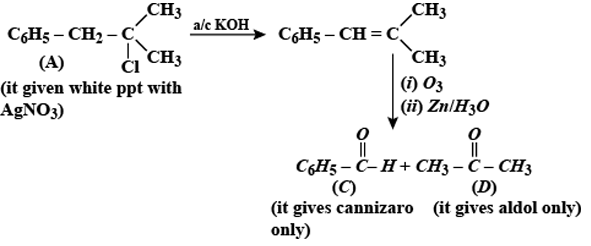
Question: A compound A with molecular formula $ C _{10}H _{13}Cl $ gives a white precipitate on adding silver nitrate solution. A on reacting with alcoholic KOH gives compound B as the main product. B on ozonolysis gives C and D. C gives Cannizaro reaction but not aldol condensation. D gives aldol condensation but not Cannizaro reaction. A is:
Options:
A) $ C_6H_5-CH_2-CH_2-CH_2-CH_2-Cl $
B) $ C_6H_5-CH_2-CH_2-\underset{Cl}{\mathop{\underset{|}{\mathop{C}}}}H-CH_3 $
C) 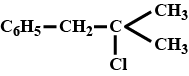
D) 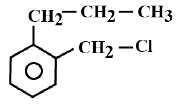
Show Answer
Answer:
Correct Answer: C
Solution:
[c] Compound A reacts with alc. KOH to give compound B which on further ozonolysis gives C (does not contains $ \alpha $ -H atom) and D (contains $ \alpha $ -H atom).This reaction sequence can be achieved by compounds in option [a] and [c]. Since compound A gives white ppt. with $ AgNO_3 $ preferable option will be [c] as tert alkyi reacts with $ AgNO_3 $ more quickly