Modern Physics Ques 182
- Half lives of two radioactive nuclei $A$ and $B$ are $10$ minutes and $20$ minutes, respectively. If initially a sample has equal number of nuclei, then after $60$ minutes, the ratio of decayed numbers of nuclei $A$ and $B$ will be
(Main 2019, 12 April II)
(a) $3: 8$
(b) $1: 8$
(c) $8: 1$
(d) $9: 8$
Show Answer
Answer:
Correct Answer: 182.(d)
Solution:
Formula:
- For substance $A$, half-life is $10 $ min, so it decays as
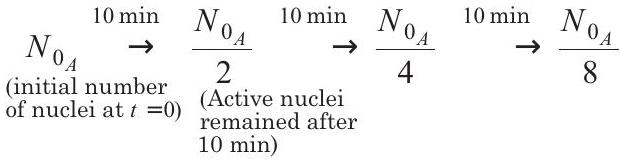
$ \begin{aligned} & \stackrel{10 \min }{\rightarrow} \frac{N _{0 _A}}{16} \stackrel{10 min}{\rightarrow} \frac{N _{0 _A}}{32} \stackrel{10 min}{\rightarrow} \frac{N _{0 _A}}{64} \end{aligned} $
$\therefore \quad $ For substance $A$, number of nuclei decayed in $60 $min is
$ N _{0 _A}-\frac{N _{0 _A}}{64}=\frac{63 N _{0 _A}}{64} $
Similarly, for substance $B$, half-life is $20 $ min, so it’s decay scheme is
$ N _{0 _B} \stackrel{20 min}{\rightarrow} \frac{N _{0 _B}}{2} \stackrel{20 min}{\rightarrow} \frac{N _{0 _B}}{4} \stackrel{20 min}{\rightarrow} \frac{N _{0 _B}}{8} $
So, number of nuclei of $B$ decayed in $60 $ min is
$ N _{0 _B}-\frac{N _{0 _B}}{8}=\frac{7}{8} N _{0 _B} $
Hence, ratio of decayed nuclei of $A$ and $B$ in $60 $ min is
$ \frac{\frac{63}{64} N _{0 _A}}{\frac{7}{8} N _{0 _B}}=\frac{9}{8} \quad\left[\because N _{0 _A}=N _{0 _B}\right] $
Alternate Solution
Number of active nuclei remained after $60 $ min can also be calculated as
$ N^{\prime}=\frac{N}{2^{T / t _{1 / 2}}} $
where, $T=60 $ min
So, for nuclei $A$,
$ N _{0 _A}^{\prime}=\frac{N _{0 _A}}{2^{\frac{60}{10}}}=\frac{N _{0 _A}}{2^{6}}=\frac{N _{0 _A}}{64} $
Similarly, for nuclei $B$,
$ N _{0 _B}^{\prime}=\frac{N _{0 _B}}{2^{\frac{60}{20}}}=\frac{N _{0 _B}}{2^{3}}=\frac{N _{0 _B}}{8} $





