Properties Of Matter Ques 63
- A column of mercury of length $10 $ $c$ $m$ is contained in the middle of a horizontal tube of length $1 $ $m$ which is closed at both the ends. The two equal lengths contain air at standard atmospheric pressure of $0.76 $ $m$ of mercury. The tube is now turned to vertical position. By what distance will the column of mercury be displaced? Assume temperature to be constant.
(1978)
Show Answer
Answer:
Correct Answer: 63.$(2.95$ $cm)$
Solution:
Formula:
- Let area of cross-section of the tube be $A$.
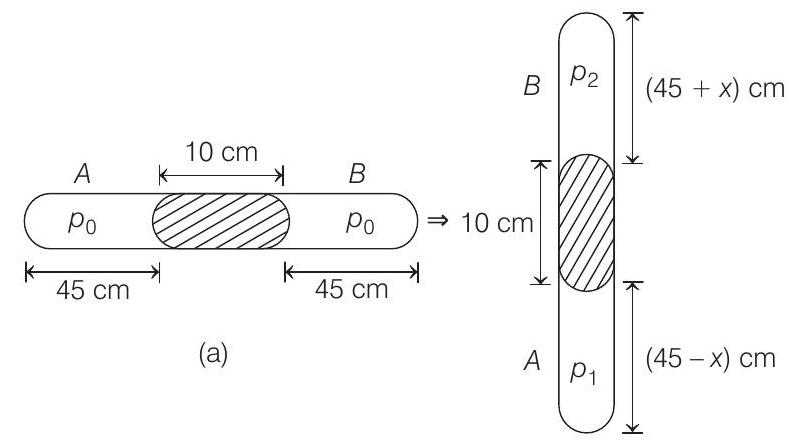
Applying $p _1 V _1=p _2 V _2$ in $A$ and $B$ we have,
$p _0(45)(A) =p _1(45-x) A $
$\text { or } \quad 76 \times 45 =p _1(45-x)$ $\quad$ …….(i)
$p _0(45)(A) =p _2(45+x) A $
$76 \times 45 =p _2(45+x)$ $\quad$ …….(ii)
From Eqs. (i) and (ii), we get
$ \left(p _1-p _2\right)=76 \times 45\left(\frac{1}{45-x}-\frac{1}{45+x}\right) $
From figure (b),
$\left(p _1-p _2\right) A=$ Weight of $10 $ $cm$ of Hg column
or $\quad p _1-p _2=$ Pressure equivalent to $10 $ $cm$ of $Hg$ column
$ 76 \times 45\left(\frac{1}{45-x}-\frac{1}{45+x}\right)=10 $
Solving this equation, we get
$ x=2.95$ $cm $





