JEE Main 12 Jan 2019 Evening Question 19
For a reaction, consider the plot of ln k versus 1/T given in the figure. If the rate constant of this reaction at 400 K is $ {10^{-5}}{s^{-1}}, $ then the rate constant at 500 K [JEE Main Online Paper Held On 12-Jan-2019 Evening]
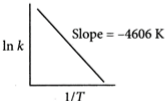
Options:
A) $ {10^{-4}}{s^{-1}} $
B) $ 4\times {10^{-4}}{s^{-1}} $
C) $ {10^{-6}}{s^{-1}} $
D) $ 2\times {10^{-4}}{s^{-1}} $
Show Answer
Answer:
Correct Answer: A
Solution:
From Arrhenius equation, $ \ln k = \ln A - \frac{E_a}{RT} $
Slope $ =\frac{-E _{a}}{R}=-\frac{4606}{R} $ or,
$ \ln \frac{k _2}{k _1}=\frac{E _{a}}{R}\left( \frac{T _2-T _1}{T _1T _2} \right) $
$ \ln =\frac{k _2}{{10^{-5}}}=4606( \frac{500-400}{500\times 400} ) $
$ \ln \frac{k _2}{{10^{-5}}}=2.303;\frac{k _2}{{10^{-5}}}=anti\ln (2.303) $
$ k _2=1\times {10^{-4}}{s^{-1}} $





