Chapter 4 Chemical Bonding and Molecular Structure
Exercise
4.1 Explain the formation of a chemical bond.
Show Answer
Answer A chemical bond is defined as an attractive force that holds the constituents (atoms, ions etc.) together in a chemical species.
Various theories have been suggested for the formation of chemical bonds such as the electronic theory, valence shell electron pair repulsion theory, valence bond theory, and molecular orbital theory.
A chemical bond formation is attributed to the tendency of a system to attain stability. It was observed that the inertness of noble gases was because of their fully filled outermost orbitals. Hence, it was postulated that the elements having incomplete outermost shells are unstable (reactive). Atoms, therefore, combine with each other and complete their respective octets or duplets to attain the stable configuration of the nearest noble gases. This combination can occur either by sharing of electrons or by transferring one or more electrons from one atom to another. The chemical bond formed as a result of sharing of electrons between atoms is called a covalent bond. An ionic bond is formed as a result of the transference of electrons from one atom to another.
4.2 Write Lewis dot symbols for atoms of the following elements : Mg, Na, B, O, N, Br.
Show Answer
Answer
Mg : There are two valence electrons in Mg atom. Hence, the Lewis dot symbol for Mg is :
$ \hspace{5cm} \boldsymbol{\overset{\bullet \ \bullet}{ \ \ Mg}}$
Na : There is only one valence electron in an atom of sodium. Hence, the Lewis dot structure is :
$ \hspace{5cm} \boldsymbol{\overset{\bullet}Na}$
B : There are 3 valence electrons in Boron atom. Hence, the Lewis dot structure is :
$ \hspace{5cm} {}^{\bullet}{\overset{\bullet\bullet}{B}}$
O : There are six valence electrons in an atom of oxygen. Hence, the Lewis dot structure is :
$ \hspace{5cm} \overset{\bullet\bullet}{{}^\bullet_\bullet{\mathrm{ \ O \ }}^\bullet_\bullet}$
N : There are five valence electrons in an atom of nitrogen. Hence, the Lewis dot structure is:
$ \hspace{5cm} \overset{\bullet\bullet}{{}^\bullet{\mathrm{ \ N \ }}^\bullet_\bullet}$
Br : There are seven valence electrons in bromine. Hence, the Lewis dot structure is :
$ \hspace{5cm} \overset{\bullet\bullet}{{}^\bullet_\bullet{\mathrm{ \ Br \ }}^\bullet_\bullet}$
4.3 Write Lewis symbols for the following atoms and ions: $\mathrm{S}$ and $\mathrm{S}^{2-} ; \mathrm{Al}$ and $\mathrm{Al}^{3+} ; \mathrm{H}$ and $\mathrm{H}^{-}$
Show Answer
Answer
(i) $S$ and $S^{2}$
The number of valence electrons in sulphur is 6 .
The Lewis dot symbol of sulphur $(S)$ is $\hspace{0.5cm}$ $ \overset{\bullet\bullet}{{}^\bullet_\bullet{\mathrm{ \ Br \ }}^\bullet_\bullet}$
The dinegative charge infers that there will be two electrons more in addition to the six valence electrons. Hence, the Lewis dot symbol of $S^{2 \cdot}$ is

(ii) $Al$ and $Al^{3+}$
The number of valence electrons in aluminium is 3 .
The Lewis dot symbol of aluminium $(Al)$ is $ \hspace{0.5cm} {}^{\bullet}{\overset{\bullet\bullet}{Al}}$
The tripositive charge on a species infers that it has donated its three electrons. Hence, the Lewis dot symbol is $[Al]^{3+}$.
(iii) $H$ and $H$
The number of valence electrons in hydrogen is 1.
The Lewis dot symbol of hydrogen $(H)$ is $ \hspace{0.5cm} \boldsymbol{\overset{\bullet}H}$
The uninegative charge infers that there will be one electron more in addition to the one valence electron. Hence, the Lewis dot symbol is $ \hspace{0.5cm} \big[\boldsymbol{\overset{\bullet \ \bullet}{ \ H}} \ \big]^-$
4.4 Draw the Lewis structures for the following molecules and ions :
$\mathrm{H}_2 \mathrm{S}, \mathrm{SiCl}_4, \mathrm{BeF}_2, \mathrm{CO}_3^{2-}$, $\mathrm{HCOOH}$
Show Answer
Answer
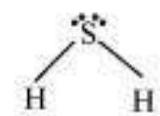
$H_2S$
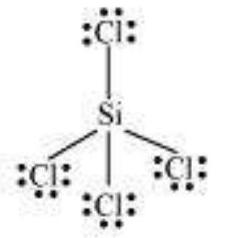
$SiCl_4$
$BeF_2$
$ \overset{ \ \ \bullet \ \bullet}{^\bullet_\bullet\underset{\bullet \ \bullet}{{F}}}{\LARGE{\boldsymbol{-}}}{Be}{\LARGE{\boldsymbol{-}}} \underset{\bullet \ \bullet \ \ \ }{\overset{\bullet \ \bullet \ \ }{F{ \ }^\bullet_\bullet}} $
$CO_3^{2-}$

4.5 Define octet rule. Write its significance and limitations.
Show Answer
Answer
The octet rule or the electronic theory of chemical bonding was developed by Kossel and Lewis. According to this rule, atoms can combine either by transfer of valence electrons from one atom to another or by sharing their valence electrons in order to attain the nearest noble gas configuration by having an octet in their valence shell.
The octet rule successfully explained the formation of chemical bonds depending upon the nature of the element.
Limitations of the octet theory:
The following are the limitations of the octet rule:
(a) The rule failed to predict the shape and relative stability of molecules.
(b) It is clear that octet rule is based upon the chemical inertness of noble gases. However, some noble gases (for example xenon and krypton) also combine with oxygen and fluorine to form a number of compounds like $\mathrm{XeF}_2, \mathrm{KrF}_2, \mathrm{XeOF}_2$ etc.
(c) Elements in and beyond the third period of the periodic table have, apart from $3 s$ and $3 p$ orbitals, $3 d$ orbitals also available for bonding. In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Obviously the octet rule does not apply in such cases.
Some of the examples of such compounds are: $\mathrm{PF}_5, \mathrm{SF}_6, \mathrm{H}_2 \mathrm{SO}_4$ and a number of coordination compounds.

(d) In molecules with an odd number of electrons like nitric oxide, NO and nitrogen dioxide, $\mathrm{NO}_2$, the octet rule is not satisfied for all the atoms.

(e) In some compounds, the number of electrons surrounding the central atom is less than eight. This is especially the case with elements having less than four valence electrons. Examples are $\mathrm{LiCl}, \mathrm{BeH}_2$ and $\mathrm{BCl}_3$.

Li, Be and B have 1, 2 and 3 valence electrons only. Some other such compounds are $\mathrm{AlCl}_3$ and $\mathrm{BF}_3$.
4.6 Write the favourable factors for the formation of ionic bond.
Show Answer
Answer
An ionic bond is formed by the transfer of one or more electrons from one atom to another. Hence, the formation of ionic bonds depends upon the ease with which neutral atoms can lose or gain electrons. Bond formation also depends upon the lattice energy of the compound formed.
Hence, favourable factors for ionic bond formation are as follows:
(i) Low ionization enthalpy of metal atom.
(ii) High electron gain enthalpy $(\Delta _{e g} H)$ of a non-metal atom.
(iii) High lattice energy of the compound formed.
4.7 Discuss the shape of the following molecules using the VSEPR model :
$\mathrm{BeCl}_2, \mathrm{BCl}_3, \mathrm{SiCl}_4, \mathrm{AsF}_5, \mathrm{H}_2 \mathrm{~S}, \mathrm{PH}_3$
Show Answer
Answer
${BeCl_2}$
The central atom has no lone pair and there are two bond pairs. i.e., $BeCl_2$ is of the type $AB_2$. Hence, it has a linear shape.
$Cl: Be: Cl$
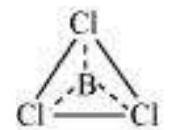
$BCl_3$
The central atom has no lone pair and there are three bond pairs. Hence, it is of the type $AB_3$. Hence, it is trigonal planar.
${SiCl_4}$
The central atom has no lone pair and there are four bond pairs. Hence, the shape of $SiCl_4$ is tetrahedral being the $AB_4$ type molecule.
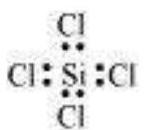
$\mathrm{AsF}_5$
The central atom has no lone pair and there are five bond pairs. Hence, $AsF_5$ is of the type $AB_5$. Therefore, the shape istrigonal bipyramidal.
$H_2 S$ :
The central atom has one lone pair and there are two bond pairs. Hence, $H_2 S$ is of the type $AB_2 E$. The shape is Bent.
н: $\ddot{s}: H$
$PH_3:$
The central atom has one lone pair and there are three bond pairs. Hence, $PH_3$ is of the $AB_3 E$ type. Therefore, the shape is trigonal pyramidal.
$H: \underset{H}{\ddot{P}}: H$
4.8 Although geometries of $\mathrm{NH}_3$ and $\mathrm{H}_2 \mathrm{O}$ molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Show Answer
Answer
The molecular geometry of $NH_3$ and $H_2 O$ can be shown as:

The central atom $(N)$ in $NH_3$ has one lone pair and there are three bond pairs. In $H_2 O$, there are two lone pairs and two bond pairs.
The two lone pairs present in the oxygen atom of $H_2 O$ molecule repels the two bond pairs. This repulsion is stronger than the repulsion between the lone pair and the three bond pairs on the nitrogen atom.
Since the repulsions on the bond pairs in $H_2 O$ molecule are greater than that in $NH_3$, the bond angle in water is less than that of ammonia.
4.9 How do you express the bond strength in terms of bond order ?
Show Answer
Answer
Bond strength represents the extent of bonding between two atoms forming a molecule. The larger the bond energy, the stronger is the bond and the greater is the bond order.
4.10 Define the bond length.
Show Answer
Answer
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule. Bond lengths are measured by spectroscopic, X-ray diffraction and electron-diffraction techniques about which you will learn in higher classes. Each atom of the bonded pair contributes to the bond length . In the case of a covalent bond, the contribution from each atom is called the covalent radius of that atom.

$R=r_A+r_B\left(R\right.$ is the bond length and $r_A$ and $r_B$ are the covalent radii of atoms $A$ and $B$ respectively)
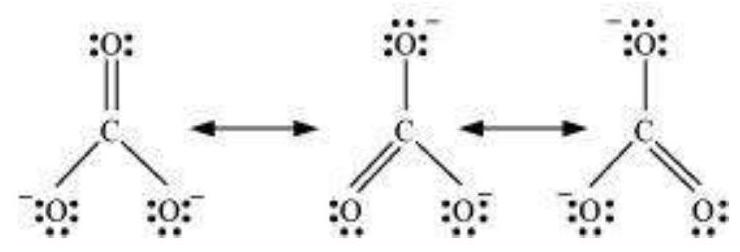
4.11 Explain the important aspects of resonance with reference to the $\mathrm{CO}_{3}^{2-}$ ion.
Show Answer
Answer
The single Lewis structure based on the presence of two single bonds and one double bond between carbon and oxygen atoms is inadequate to represent the molecule accurately as it represents unequal bonds. According to the experimental findings, all carbon to oxygen bonds in $\mathrm{CO}_3^{2-}$ are equivalent. Therefore the carbonate ion is best described as a resonance hybrid of the canonical forms as shown below.
4.12 $ \mathrm{H}_3 \mathrm{PO}_3$ can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing $\mathrm{H}_3 \mathrm{PO}_3$ ? If not, give reasons for the same.

Show Answer
Answer
The given structures cannot be taken as the canonical forms of the resonance hybrid of $H_3 PO_3$ because the positions of the atoms have changed.
4.13 Write the resonance structures for $\mathrm{SO}_3, \mathrm{NO}_2$ and $\mathrm{NO}_3^{-}$.
Show Answer
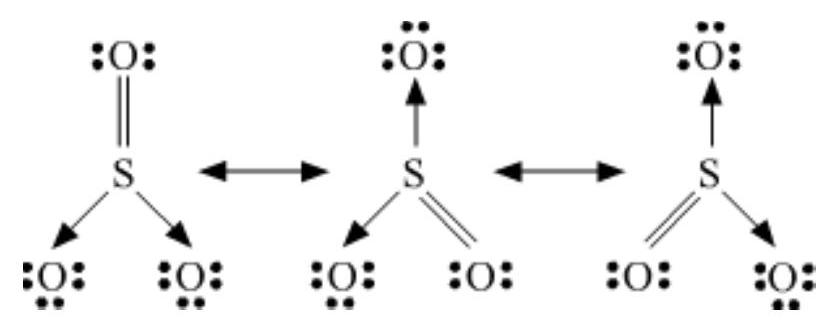
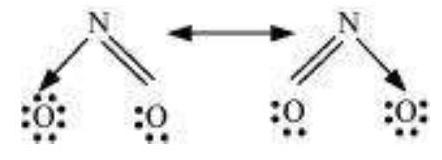
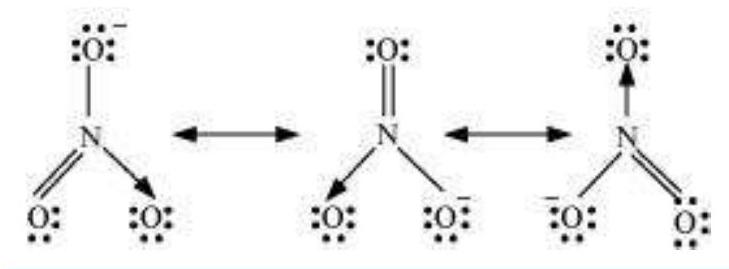
Answer
The resonance structures are:
(a) $SO_3$ :
(b) $NO_2$ :
(c) $NO_3^{-}$:
4.14 Use Lewis symbols to show electron transfer between the following atoms to form cations and anions : (a) $\mathrm{K}$ and $\mathrm{S}$ (b) $\mathrm{Ca}$ and $\mathrm{O}$ (c) $\mathrm{Al}$ and $\mathrm{N}$.
Show Answer
Answer
(a) $K$ and $S$ -
The electronic configurations of $K$ and $S$ are as follows:
$K : 2, 8, 8, 1$
$S : 2,8,6$
Sulphur (S) requires 2 more electrons to complete its octet. Potassium (K) requires one electron more than the nearest noble gas i.e., Argon. Hence, the electron transfer can be shown as:

(b) Ca and O -
The electronic configurations of $Ca$ and $O$ are as follows :
$Ca : 2,8,8,2$
$O : 2,6$
Oxygen requires two electrons more to complete its octet, whereas calcium has two electrons more than the nearest noble gas i.e., Argon. Hence, the electron transfer takes place as:

(c) Al and $N$ :
The electronic configurations of $Al$ and $N$ are as follows:
$Al : 2, 8, 3$
$N : 2,5$
Nitrogen is three electrons short of the nearest noble gas (Neon), whereas aluminium has three electrons more than Neon. Hence, the electron transference can be shown as:

4.15 Although both $\mathrm{CO}_2$ and $\mathrm{H}_2 \mathrm{O}$ are triatomic molecules, the shape of $\mathrm{H}_2 \mathrm{O}$ molecule is bent while that of $\mathrm{CO}_2$ is linear. Explain this on the basis of dipole moment.
Show Answer
Answer
According to experimental results, the dipole moment of carbon dioxide is zero. This is possible only if the molecule is linear so that the dipole moments of $C$-O bonds are equal and opposite to nullify each other.

Resultant $\mu=0$ D
$H_2 O$, on the other hand, has a dipole moment value of $1.84 \hspace{0.8mm} D$ (though it is a triatomic molecule as $CO_2$ ). The value of the dipole moment suggests that the structure of $H_2 O$ molecule is bent where the dipole moment of $O-H$ bonds are unequal.

4.16 Write the significance/applications of dipole moment.
Show Answer
Answer
In heteronuclear molecules, polarization arises due to a difference in the electronegativities of the constituents of atoms. As a result, one end of the molecule acquires a positive charge while the other end becomes negative. Hence, a molecule is said to possess a dipole.
The product of the magnitude of the charge and the distance between the centres of positive-negative charges is called the dipole moment $ \mu $ of the molecule.
Dipole moment $(\mu)=$ charge $(Q) \times$ distance of separation $(r)$
Further dipole momer and by convention it is arrow with tail on the head pointing towards But in chemistry presen is represented by the c put on Lewis structure cross is on positive end a negative end. For exampl of HF may be represented as :

This arrow symbolises the direction of the shift of electron density in the molecule. Note that the direction of crossed arrow is opposite to the conventional direction of dipole moment vector.
4.17 Define electronegativity. How does it differ from electron gain enthalpy?
Show Answer
Answer
Electronegativity is the ability of an atom in a chemical compound to attract a bond pair of electrons towards itself.
Electronegativity of any given element is not constant. It varies according to the element to which it is bound. It is not a measurable quantity. It is only a relative number.
On the other hand, electron gain enthalpy is the enthalpy change that takes place when an electron is added to a neutral gaseous atom to form an anion. It can be negative or positive depending upon whether the electron is added or removed. An element has a constant value of the electron gain enthalpy that can be measured experimentally.
4.18 Explain with the help of suitable example polar covalent bond.
Show Answer
Answer
When two dissimilar atoms having different electronegativities combine to form a covalent bond, the bond pair of electrons is not shared equally. The bond pair shifts towards the nucleus of the atom having greater electronegativity. As a result, electron distribution gets distorted and the electron cloud is displaced towards the electronegative atom.
As a result, the electronegative atom becomes slightly negatively charged while the other atom becomes slightly positively charged. Thus, opposite poles are developed in the molecule and this type of a bond is called a polar covalent bond.
for example $HCl$ ,contains a polar covalent bond. Chlorine atom is more electronegative than hydrogen atom. Hence, the bond pair lies towards chlorine and therefore, it acquires a partial negative charge.
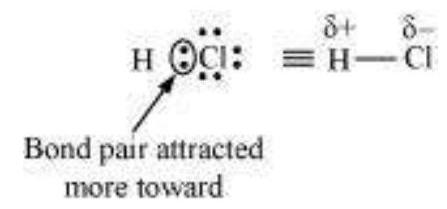
4.19 Arrange the bonds in order of increasing ionic character in the molecules: $\mathrm{LiF}, \mathrm{K}_2 \mathrm{O}$, $\mathrm{N}_2, \mathrm{SO}_2$ and $\mathrm{ClF}_3$.
Show Answer
Answer
The ionic character in a molecule is dependent upon the electronegativity difference between the constituting atoms. The greater the difference, the greater will be the ionic character of the molecule.
On this basis, the order of increasing ionic character in the given molecules is
$N_2 < SO_2 < ClF_3 < K_2 O < LiF $.
4.20 The skeletal structure of $\mathrm{CH}_{3} \mathrm{COOH}$ as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.

Show Answer
Answer
The correct Lewis structure for acetic acid is as follows :

4.21 Apart from tetrahedral geometry, another possible geometry for $\mathrm{CH_4}$ is square planar with the four $\mathrm{H}$ atoms at the corners of the square and the $\mathrm{C}$ atom at its centre. Explain why $\mathrm{CH_4}$ is not square planar?
Show Answer
Answer
Electronic configuration of carbon atom :
$ _6 C: 1 s^{2} 2 s^{2} 2 p^{2}$
In the excited state, the orbital picture of carbon can be represented as :
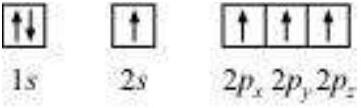
Hence, carbon atom undergoes $s p^{3}$ hybridization in $CH_4$ molecule and takes a tetrahedral shape.
For a square planar shape, the hybridization of the central atom has to be $d s p^{2}$. However, an atom of carbon does not have $d$-orbitalsto undergo $d s p^{2}$ hybridization. Hence, the structure of $CH_4$ cannot be square planar.
Moreover, with a bond angle of $90^{\circ}$ in square planar, the stability of $CH_4$ will be very less because of the repulsion existing between the bond pairs. Hence, VSEPR theory also supports a tetrahedral structure for $CH_4$.
4.22 Explain why $\mathrm{BeH_2}$ molecule has a zero dipole moment although the $\mathrm{Be}-\mathrm{H}$ bonds are polar.
Show Answer
Answer
The Lewis structure for $BeH_2$ is as follows:
$H: Be: H$
There is no lone pair at the central atom (Be) and there are two bond pairs. Hence, $BeH_2$ is of the type $AB_2$. It has a linear structure.

Dipole moments of each $H-Be$ bond are equal and are in opposite directions. Therefore, they nullify each other. Hence, $BeH_2$ molecule has zero dipole moment.
4.23 Which out of $\mathrm{NH_3}$ and $\mathrm{NF_3}$ has higher dipole moment and why ?
Show Answer
Answer
Let us study an interesting case of $\mathrm{NH}_3$ and $\mathrm{NF}_3$ molecule. Both the molecules have pyramidal shape with a lone pair of electrons on nitrogen atom. Although fluorine is more electronegative than nitrogen, the resultant dipole moment of $\mathrm{NH}_3\left(4.90 \times 10^{-30} \mathrm{C} \mathrm{m}\right)$ is greater than that of $\mathrm{NF}_3\left(0.8 \times 10^{-30} \mathrm{C} \mathrm{m}\right)$. This is because, in case of $\mathrm{NH}_3$ the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the $\mathrm{N}-\mathrm{H}$ bonds, whereas in $\mathrm{NF}_3$ the orbital dipole is in the direction opposite to the resultant dipole moment of the three $\mathrm{N}-\mathrm{F}$ bonds. The orbital dipole because of lone pair decreases the effect of the resultant $\mathrm{N}-\mathrm{F}$ bond moments, which results in the low dipole moment of $\mathrm{NF}_3$ as represented below :

Hence, the net dipole moment of $NF_3$ is less than that of $NH_3$.
4.24 What is meant by hybridisation of atomic orbitals? Describe the shapes of $s p, s p^{2}$, $s p^{3}$ hybrid orbitals.
Show Answer
Answer
Hybridization is defined as an intermixing of a set of atomic orbitals of slightly different energies, thereby forming a new set of orbitals having equivalent energies and shapes.
For example, one $2 s$-orbital hybridizes with two $2 p$-orbitals of carbon to form three new $s p^{2}$ hybrid orbitals.
These hybrid orbitals have minimum repulsion between their electron pairs and thus, are more stable. Hybridization helps indicate the geometry of the molecule.
Shape of $s p$ hybrid orbitals: $s p$ hybrid orbitals have a linear shape. They are formed by the intermixing of $s$ and porbitals as:

$s p$-hybrids
Shape of $s p^{2}$ hybrid orbitals :
$s p^{2}$ hybrid orbitals are formed as a result of the intermixing of one s-orbital and two $2 p$-orbitals. The hybrid orbitals are oriented in a trigonal planar arrangement as:

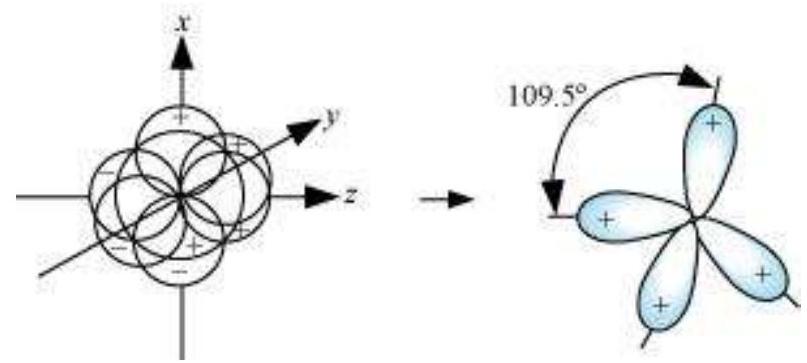
Shape of $s p^{3}$ hybrid orbitals:
Four $s p^{3}$ hybrid orbitals are formed by intermixing one s-orbital with three $p$-orbitals. The four $s p^{3}$ hybrid orbitals are arranged in the form of a tetrahedron as:
4.25 Describe the change in hybridisation (if any) of the Al atom in the following reaction.
$\mathrm{AlCl_3}+\mathrm{Cl}^{-} \rightarrow \mathrm{AlCl_4}^{-}$
Show Answer
Answer
To form $AlCl_4^{-}$, the empty $3 p_z$ orbital also gets involved and the hybridization changes from $s p^{2}$ to $s p^{3}$. As a result, the shape gets changed to tetrahedral.

4.26 Is there any change in the hybridisation of $\mathrm{B}$ and $\mathrm{N}$ atoms as a result of the following reaction?
$\mathrm{BF_3}+\mathrm{NH_3} \rightarrow \mathrm{F_3} \mathrm{B} - \mathrm{NH_3}$
Show Answer
Answer
In $\mathrm{BF}_3, \mathrm{~B}$ is $s p^2$ hybridised and in $\mathrm{NH}_3, \mathrm{~N}$ is $s p^3$ hybridised. After the reaction, hybridsation of $B$ changes to $s p^3$ but that of $N$ remains unchanged.
4.27 Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in $\mathrm{C_2} \mathrm{H_4}$ and $\mathrm{C_2} \mathrm{H_2}$ molecules.
Show Answer
Answer
$ C_2 H_4$ :
In the formation of ethene molecule, one of the $s p^2$ hybrid orbitals of carbon atom overlaps axially with $s p^2$ hybridised orbital of another carbon atom to form $\mathrm{C}-\mathrm{C}$ sigma bond. While the other two $s p^2$ hybrid orbitals of each carbon atom are used for making $s p^2-s$ sigma bond with two hydrogen atoms. The unhybridised orbital $\left(2 p_x\right.$ or $2 p_y$ ) of one carbon atom overlaps sidewise with the similar orbital of the other carbon atom to form weak $\pi$ bond, which consists of two equal electron clouds distributed above and below the plane of carbon and hydrogen atoms.

$ C_2 H_2$ :
In the formation of ethyne molecule, both the carbon atoms undergo $s p$-hybridisation having two unhybridised orbital ie., $2 p_y$ and $2 p_{\mathrm{x}}$.
One $s p$ hybrid orbital of one carbon atom overlaps axially with $s p$ hybrid orbital of the other carbon atom to form $\mathrm{C}-\mathrm{C}$ sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half filled $s$ orbital of hydrogen atoms forming $\sigma$ bonds. Each of the two unhybridised $p$ orbitals of both the carbon atoms overlaps sidewise to form two $\pi$ bonds between the carbon atoms. So the triple bond between the two carbon atoms is made up of one sigma and two pi bonds as shown in Fig.

4.28 What is the total number of sigma and pi Bonds in the following molecules?
(a) $\mathrm{C_2} \mathrm{H_2}$ (b) $\mathrm{C_2} \mathrm{H_4}$
Show Answer
Answer
(a) $\mathrm{C_2} \mathrm{H_2}$
One $s p$ hybrid orbital of one carbon atom overlaps axially with $s p$ hybrid orbital of the other carbon atom to form $\mathrm{C}-\mathrm{C}$ sigma bond, while the other hybridised orbital of each carbon atom overlaps axially with the half filled $s$ orbital of hydrogen atoms forming $\sigma$ bonds. Each of the two unhybridised $p$ orbitals of both the carbon atoms overlaps sidewise to form two $\pi$ bonds between the carbon atoms. So the triple bond between the two carbon atoms is made up of one sigma and two pi bonds as shown in Fig.

Hence, there are three sigma and two pi-bonds in $C_2 H_2$.
(b) $\mathrm{C_2} \mathrm{H_4}$
Thus, in ethene molecule, the carboncarbon bond consists of one $s p^2-s p^2$ sigma bond and one pi ( $\pi$ ) bond between $p$ orbitals which are not used in the hybridisation and are perpendicular to the plane of molecule; the bond length 134 pm . The $\mathrm{C}-\mathrm{H}$ bond is $s p^2-s$ sigma with bond length 108 pm . The $\mathrm{H}-\mathrm{C}-\mathrm{H}$ bond angle is $117.6^{\circ}$ while the $\mathrm{H}-\mathrm{C}-\mathrm{C}$ angle is $121^{\circ}$. The formation of sigma and pi bonds in ethene is shown in Fig. 4.15.

Hence, there are five sigma bonds and two pi-bond in $C_2 H_4$.
4.29 Considering $x$-axis as the internuclear axis which out of the following will not form a sigma bond and why? (a) $1 \mathrm{~s}$ and $1 \mathrm{~s}$ (b) $1 \mathrm{~s}$ and $2 p_{\mathrm{x}}$; (c) $2 p_{\mathrm{y}}$ and $2 p_{\mathrm{y}}$ (d) $1 s$ and $2 s$.
Show Answer
Answer
$2 p_y$ and $2 p_y$ orbitals will not a form a sigma bond. Taking $x$-axis as the internuclear axis, $2 p_y$ and $2 p_y$ orbitals will undergo lateral overlapping, thereby forming a pi ( $\pi$ ) bond.
4.30 Which hybrid orbitals are used by carbon atoms in the following molecules?
(a) $\mathrm{CH_3}-\mathrm{CH_3}$;
(b) $\mathrm{CH_3}-\mathrm{CH}=\mathrm{CH_2}$;
(c) $\mathrm{CH_3}-\mathrm{CH_2}-\mathrm{OH}$;
(d) $\mathrm{CH_3}-\mathrm{CHO}$;
(e) $\mathrm{CH_3} \mathrm{COOH}$;
Show Answer
Answer
(a) $\mathrm{CH}_3-\mathrm{CH}_3$
Both the carbon atoms are $\mathrm s{p}^3$ hybridized.
(b) $\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}_2$
The carbon atom of methyl group is $\mathrm s{p}^3$ hybridized whereas other two carbon atoms are $\mathrm s{p}^2$ hybridized.
(c) $\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{OH}$
Both the carbon atoms are $\mathrm s{p}^3$ hybridized.
(d) $\mathrm{CH}_3-\mathrm{CHO}$
The carbon atom of methyl group is $\mathrm s{p}^3$ hybridized whereas the other carbon atom is $\mathrm s{p}^2$ hybridized.
(e) $\mathrm{CH}_3 \mathrm{COOH}$
The carbon atom of methyl group is $\mathrm s{p}^3$ hybridized whereas the other carbon atom is $\mathrm s{p}^2$ hybridized.
4.31 What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one exmaple of each type.
Show Answer
Answer
When two atoms combine by sharing their one or more valence electrons, a covalent bond is formed between them.
The shared pairs of electrons present between the bonded atoms are called bond pairs. All valence electrons may not participate in bonding. The electron pairs that do not participate in bonding are called lone pairs of electrons.
For example, in $C_2 H_6$ (ethane), there are seven bond pairs but no lone pair present.
$\mathrm{\boldsymbol{{H-}\overset{\overset{\huge{H}}{|}}{\underset{\underset{\huge{H}}{|}}{C}}-\overset{\overset{\huge{H}}{|}}{\underset{\underset{\huge{H}}{|}}{{C}}}{-H}}}$
In $H_2 O$, there are two bond pairs and two lone pairs on the central atom (oxygen).

4.32 Distinguish between a sigma and a pi bond.
Show Answer
Answer
The following are the differences between sigma and pi-bonds :
| Sigma ( $ \sigma $) Bond | Pi $(\pi)$ Bond |
|---|---|
| (a) It is formed by the end to end overlap of orbitals. | It is formed by the lateral overlap of orbitals. |
| (b) The orbitals involved in the overlapping are s -s, s- $p$, or p - $p$. |
These bonds are formed by the overlap of $p - p$ orbitals only. |
| (c) It is a strong bond. | It is weak bond. |
| (d) The electron cloud is symmetrical about the line joining the two nuclei. |
The electron cloud is not symmetrical. |
| (e) It consists of one electron cloud, which is symmetrical about the internuclear axis. |
There are two electron clouds lying above and below the plane of the atomic nuclei. |
| (f) Free rotation about $\sigma $ bonds is possible. | Rotation is restricted in case of pi-bonds. |
4.33 Explain the formation of $\mathrm{H_2}$ molecule on the basis of valence bond theory.
Show Answer
Answer
Let us assume that two hydrogen atoms ( $A$ and $B$ ) with nuclei $(N_A$ and $N_B)$ and electrons $(e_A$ and $e_B)$ are taken to undergo a reaction to form a hydrogen molecule.
When $A$ and $B$ are at a large distance, there is no interaction between them. As they begin to approach each other, the attractive and repulsive forces start operating.
Attractive force arises between:
(a) Nucleus of one atom and its own electron i.e., $N_A-e_A$ and $N_B-e_B$.
(b) Nucleus of one atom and electron of another atom i.e., $N_A-e_B$ and $N_B-e_A$
Repulsive force arises between:
(a) Electrons of two atoms i.e., $e_A-e_B$
(b) Nuclei of two atoms i.e., $N_A-N_B$.
The force of attraction brings the two atoms together, whereas the force of repulsion tends to push them apart.

The magnitude of new attractive force is more than the new repulsive forces. As a result, two atoms approach each other and potential energy decreases. Ultimately a stage is reached where the net force of attraction balances the force of repulsion and system acquires minimum energy. At this stage two hydrogen atoms are said to be bonded together to form a stable molecule having the bond length of 74 pm. This leads to the formation of a dihydrogen molecule.
4.34 Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals.
Show Answer
Answer
The given conditions should be satisfied by atomic orbitals to form molecular orbitals:
-
The combining atomic orbitals must have the same or nearly the same energy. This means that $1 s$ orbital can combine with another $1 s$ orbital but not with $2 s$ orbital because the energy of $2 s$ orbital is appreciably higher than that of 1 s orbital. This is not true if the atoms are very different.
-
The combining atomic orbitals must have the same symmetry about the molecular axis. By convention z-axis is taken as the molecular axis. It is important to note that atomic orbitals having same or nearly the same energy will not combine if they do not have the same symmetry. For example, $2 p_z$ orbital of one atom can combine with $2 p_z$ orbital of the other atom but not with the $2 p_x$ or $2 p_y$ orbitals because of their different symmetries.
-
The combining atomic orbitals must overlap to the maximum extent. Greater the extent of overlap, the greater will be the electron-density between the nuclei of a molecular orbital.
4.35 Use molecular orbital theory to explain why the $\mathrm{Be_2}$ molecule does not exist.
Show Answer
Answer
The electronic configuration of Beryllium is $1 s^{2} 2 s^{2}$.
The molecular orbital electronic configuration for $Be_2$ molecule can be written as:
$(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2$
Hence, the bond order for $Be_2$ is $\dfrac{1}{2}(N_b-N_a)$.
Where,
$N_b=$ Number of electrons in bonding orbitals
$N_a=$ Number of electrons in anti-bonding orbitals
$\therefore$ Bond order of $Be_2=\dfrac{1}{2}(4-4)=0$
A negative or zero bond order means that the molecule is unstable.
Hence, $Be_2$ molecule does not exist.
4.36 Compare the relative stability of the following species and indicate their magnetic properties;
$\mathrm{O_2}, \mathrm{O}_2^{+}, \mathrm{O}_2^{-}$(superoxide), $\mathrm{O}_2^{2-}$ (peroxide)
Show Answer
Answer
There are 16 electrons in a molecule of dioxygen, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as :
$\begin{aligned} & \mathrm{O}_2:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^1=\pi^* 2 p_y^1\right)\end{aligned}$
From the electronic configuration of $\mathrm{O}_2$ molecule it is clear that ten electrons are present in bonding molecular orbitals and six electrons are present in antibonding molecular orbitals. Its bond order, therefore, is
Bond order $=\dfrac{1}{2}\left[\mathrm{~N}{\mathrm{b}}-\mathrm{N}{\mathrm{a}}\right]=\dfrac{1}{2}[10-6]=2$
Similarly, the electronic configuration of $O_2^{+}$ can be written as :
$\begin{aligned} & \mathrm{O}_2^{+}:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^1=\pi^* 2 p_y^1\right)\end{aligned}$
$N_b=10$
$N_a=5$
Bond order of $O_2^{+}=\dfrac{1}{2}(10-5)$
$ \hspace{3.2cm}=2.5$
Electronic configuration of $O_2^{-}$ ion will be :
$\begin{aligned} & \mathrm{O}_2^{-}:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^2=\pi^* 2 p_y^1\right)\end{aligned}$
$N_b=10$
$N_a=7$
Bond order of $O_2^{-}=\dfrac{1}{2}(10-7)$
$ \hspace{3.2cm} =1.5$
Electronic configuration of $O_2^{2-}$ ion will be :
$\begin{aligned} & \mathrm{O}_2^{2-}:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^2=\pi^* 2 p_y^2\right)\end{aligned}$
$N_b=10$
$N_a=8$
Bond order of $O_2^{2-}=\dfrac{1}{2}(10-8)$
$ \hspace{3.4cm} =1$
Bond dissociation energy is directly proportional to bond order. Thus, the higher the bond order, the greater will be the stability. On this basis, the order of stability is $ O_2^{+} > O_2 > O_2^{-} > O_2^{2-} $.
4.37 Write the significance of a plus and a minus sign shown in representing the orbitals.
Show Answer
Answer
Molecular orbitals are represented by wave functions. A plus sign in an orbital indicates a positive wave function while a minus sign in an orbital represents a negative wave function.
4.38 Describe the hybridisation in case of $\mathrm{PCl_5}$. Why are the axial bonds longer as compared to equatorial bonds?
Show Answer
Answer
The ground state and the excited state outer electronic configurations of phosphorus $(Z=15)$ are represented below.

Now the five orbitals (ie., one s, three $p$ and one $d$ orbitals) are available for hybridisation to yield a set of five $s p^3 d$ hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal.

It should be noted that all the bond angles in trigonal bipyramidal geometry are not equivalent. In $\mathrm{PCl}_5$ the five $s p^3 d$ orbitals of phosphorus overlap with the singly occupied $p$ orbitals of chlorine atoms to form five $\mathrm{P}-\mathrm{Cl}$ sigma bonds.
Three $\mathrm{P}-\mathrm{Cl}$ bond lie in one plane and make an angle of $120^{\circ}$ with each other; these bonds are termed as equatorial bonds.
The remaining two $\mathrm{P}-\mathrm{Cl}$ bonds-one lying above and the other lying below the equatorial plane, make an angle of $90^{\circ}$ with the plane. These bonds are called axial bonds.
As the axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than the equatorial bonds; which makes $\mathrm{PCl}_5$ molecule more reactive.
4.39 Define hydrogen bond. Is it weaker or stronger than the van der Waals forces?
Show Answer
Answer
A hydrogen bond is defined as an attractive force acting between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule (may be of the same kind).
Due to a difference between electronegativities, the bond pair between hydrogen and the electronegative atom gets drifted far away from the hydrogen atom. As a result, a hydrogen atom becomes electropositive with respect to the other atom and acquires a positive charge.
$\mathrm{H}^{\delta+}-\mathrm{X}^{\delta-}—\mathrm{H}^{\delta+}-\mathrm{X}^{\delta-}—\mathrm{H}^{\delta+}-\mathrm{X}^{\delta-}$
The magnitude of H-bonding depends on the physical state of the compound. It is maximum in the solid state and minimum in the gaseous state. Thus, the hydrogen bonds have strong influence on the structure and properties of the compounds.
There are two types of $H$-bonds:
(1) Intermolecular hydrogen bond : It is formed between two different molecules of the same or different compounds. For example, H -bond in case of HF molecule, alcohol or water molecules, etc.
(2) Intramolecular hydrogen bond : It is formed when hydrogen atom is in between the two highly electronegative ( $\mathrm{F}, \mathrm{O}, \mathrm{N}$ ) atoms present within the same molecule. For example, in o-nitrophenol the hydrogen is in between the two oxygen atoms.

Hydrogen bonds are stronger than Van der Walls forces since hydrogen bonds are regarded as an extreme form of dipole-dipole interaction.
4.40 What is meant by the term bond order? Calculate the bond order of : $\mathrm{N_2}, \mathrm{O_2}, \mathrm{O_2}^{+}$ and $\mathrm{O_2}^{-}$.
Show Answer
Answer
Bond order is defined as one half of the difference between the number of electrons present in the bonding and antibonding orbitals of a molecule.
If $N_a$ is equal to the number of electrons in an anti-bonding orbital, then $N_b$ is equal to the number of electrons in a bonding orbital.
Bond order $=\dfrac{1}{2}(N_b-N_a)$
If $N_b>N_a$, then the molecule is said be stable. However, if $N_b \leq N_a$, then the molecule is considered to be unstable.
Bond order of $N_2$ can be calculated from its electronic configuration as:
$\begin{aligned} & \mathrm{O}_2:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\end{aligned}$
Number of bonding electrons, $N_b=10$
Number of anti-bonding electrons, $N_a=4$
Bond order of nitrogen molecule $=\dfrac{1}{2}(10-4)$
$ \hspace{5.7cm}=3$
There are 16 electrons in a molecule of dioxygen, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as :
$\begin{aligned} & \mathrm{O}_2:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^1=\pi^* 2 p_y^1\right)\end{aligned}$
From the electronic configuration of $\mathrm{O}_2$ molecule it is clear that ten electrons are present in bonding molecular orbitals and six electrons are present in antibonding molecular orbitals. Its bond order, therefore, is
Bond order $=\dfrac{1}{2}\left[\mathrm{~N}{\mathrm{b}}-\mathrm{N}{\mathrm{a}}\right]=\dfrac{1}{2}[10-6]=2$
Similarly, the electronic configuration of $O_2^{+}$ can be written as :
$\begin{aligned} & \mathrm{O}_2^{+}:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^1=\pi^* 2 p_y^1\right)\end{aligned}$
$N_b=10$
$N_a=5$
Bond order of $O_2^{+}=\dfrac{1}{2}(10-5)$
$ \hspace{3.2cm}=2.5$
Electronic configuration of $O_2^{-}$ ion will be :
$\begin{aligned} & \mathrm{O}_2^{-}:(\sigma 1 s)^2\left(\sigma^* 1 s\right)^2(\sigma 2 s)^2(\sigma * 2 s)^2\left(\sigma 2 \mathrm{p}_z\right)^2 \left(\pi 2 p_x^2=\pi 2 p_y^2\right)\left(\pi^* 2 p_x^2=\pi^* 2 p_y^1\right)\end{aligned}$
$N_b=10$
$N_a=7$
Bond order of $O_2^{-}=\dfrac{1}{2}(10-7)$
$ \hspace{3.2cm} =1.5$





