Unit 13 Amines (Exercises)
Exercises
13.1 Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) $\left({CH_3}\right)_{2} {CHNH_2}$
(ii) ${CH_3}\left({CH_2}\right)_{2} {NH_2}$
(iii) ${CH_3} {NHCH}\left({CH_3}\right)_{2}$
(iv) $\left({CH_3}\right)_{3} {CNH_2}$
(v) ${C_6} {H_5} {NHCH_3}$
(vi) $\left({CH_3} {CH_2}\right)_{2} {NCH_3}$
(vii) $m-{BrC_6} {H_4} {NH_2}$
Show Answer
Answer
(i) 1-Methylethanamine ( $1^{\circ}$ amine)
(ii) Propan-1-amine ( $1^{\circ}$ amine)
(iii) N-Methyl-2-methylethanamine ( $2^{\circ}$ amine)
(iv) 2-Methylpropan-2-amine ( $1^{\circ}$ amine)
(v) N-Methylbenzamine or ${N}$-methylaniline ($2^{\circ}$ amine)
(vi) ${N}$-Ethyl- ${N}$-methylethanamine ( $3^{\circ}$ amine)
(vii) 3-Bromobenzenamine or 3-bromoaniline ( $1^{\circ}$ amine)
13.2 Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and ${N}$-methylaniline.
Show Answer
Answer
(i) Methylamine and dimethylamine can be distinguished by the carbylamine test.
Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form foul-smelling isocyanides or carbylamines. Methylamine (being an aliphatic primary amine) gives a positive carbylamine test, but dimethylamine does not.
(ii) Secondary and tertiary amines can be distinguished by allowing them to react with Hinsberg’s reagent (benzenesulphonyl chloride, ${C_6} {H_5} {SO_2} {Cl}$ ).
Secondary amines react with Hinsberg’s reagent to form a product that is insoluble in an alkali. For example, N, N - diethylamine reacts with Hinsberg’s reagent to form N, N diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg’s reagent.
(iii) Ethylamine and aniline can be distinguished using the azo-dye test.
A dye is obtained when aromatic amines react with ${HNO_2}\left({NaNO_2}+\right.$ dil. $\left.{HCl}\right)$ at $0-5^{\circ} {C}$, followed by a reaction with the alkaline solution of 2-naphthol. The dye is usually yellow, red, or orange in colour. Aliphatic amines give a brisk effervescence due (to the evolution of ${N_2}$ gas) under similar conditions.
(iv) Aniline and benzylamine can be distinguished by their reactions with the help of nitrous acid, which is prepared in situ from a mineral acid and sodium nitrite. Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
On the other hand, aniline reacts with ${HNO_2}$ at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
(v) Aniline and N-methylaniline can be distinguished using the Carbylamine test.
Primary amines, on heating with chloroform and ethanolic potassium hydroxide, form foul-smelling isocyanides or carbylamines. Aniline, being an aromatic primary amine, gives positive carbylamine test. However, N-methylaniline, being a secondary amine does not.
13.3 Account for the following:
(i) ${pK_b}$ of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is $O-$ and $p$ - directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Show Answer
Answer
(i) ${p} K_{b}$ of aniline is more than that of methylamine :

Aniline undergoes resonance and as a result, the electrons on the ${N}$-atom are delocalized over the benzene ring. Therefore, the electrons on the ${N}$-atom are less available to donate.

On the other hand, in case of methylamine (due to the $+{I}$ effect of methyl group), the electron density on the ${N}$-atom is increased. As a result, aniline is less basic than methylamine. Thus, $p K_{b}$ of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not :
Ethylamine when added to water forms intermolecular ${H}$ - bonds with water. Hence, it is soluble in water.

But aniline does not undergo ${H}$ - bonding with water to a very large extent due to the presence of a large hydrophobic - ${C_6} {H_5}$ group. Hence, aniline is insoluble in water.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide :
Due to the $+{I}$ effect of - ${CH_3}$ group, methylamine is more basic than water. Therefore, in water, methylamine produces ${OH^-}$ ions by accepting ${H}^{+}$ ions from water.
$ {CH_3}-{NH_2}+{H}-{OH} \longrightarrow {CH_3}-\stackrel{+}{{N}} {H_3}+{OH}^{-} $
Ferric chloride $\left({FeCl_3}\right)$ dissociates in water to form ${Fe}^{3+}$ and ${Cl^-}$ ions.
$ {FeCl_3} \longrightarrow {Fe}^{3+}+3 {Cl}^{-} $
Then, ${OH^-}$ ion reacts with ${Fe}^{3+}$ ion to form a precipitate of hydrated ferric oxide.
$ 2 {Fe}^{3+}+6 {OH}^{-} \longrightarrow \underset{{\substack{\text{Hydrated}\\ \text{ferric oxide}}}}{{Fe_2} {O_3} \cdot 3 {H_2} {O}} $
(iv) Although amino group is $o-, p$ - directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of $\boldsymbol{m}$-nitroaniline:
Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ion (which is meta-directing).

For this reason, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction :
A Friedel-Crafts reaction is carried out in the presence of ${AlCl_3}$. But ${AlCl_3}$ is acidic in nature, while aniline is a strong base. Thus, aniline reacts with ${AlCl_3}$ to form a salt (as shown in the following equation).

Due to the positive charge on the ${N}$-atom, electrophilic substitution in the benzene ring is deactivated. Hence, aniline does not undergo the Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines:
The diazonium ion undergoes resonance as shown below:

This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines :
Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine. Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.

13.4 Arrange the following:
(i) In decreasing order of the ${p} K_{b}$ values:
${C_2} {H_5} {NH_2}, {C_6} {H_5} {NHCH_3},\left({C_2} {H_5}\right)_{2} {NH}$ and ${C_6} {H_5} {NH_2}$
(ii) In increasing order of basic strength:
${C_6} {H_5} {NH_2}, {C_6} {H_5} {~N}\left({CH_3}\right)_{2},\left({C_2} {H_5}\right)_2 {NH}$ and ${CH_3} {NH_2}$
(iii) In increasing order of basic strength:
(a) Aniline, $p$-nitroaniline and $p$-toluidine
(b) ${C_6} {H_5} {NH_2}, {C_6} {H_5} {NHCH_3}, {C_6} {H_5} {CH_2} {NH_2}$.
(iv) In decreasing order of basic strength in gas phase:
${C_2} {H_5} {NH_2},\left({C_2} {H_5}\right)_2 {NH},\left({C_2} {H_5}\right)_3 {~N}$ and ${NH_3}$
(v) In increasing order of boiling point:
${C_2} {H_5} {OH},\left({CH_3}\right)_{2} {NH}, {C_2} {H_5} {NH_2}$
(vi) In increasing order of solubility in water:
${C_6} {H_5} {NH_2},\left({C_2} {H_5}\right)_{2} {NH}, {C_2} {H_5} {NH_2}$.
Show Answer
Answer
(i) In ${C _2} {H _5} {NH _2}$, only one $-{C _2} {H _5}$ group is present while in $\left({C _2} {H _5}\right) _{2} {NH}$, two $-{C _2} {H _5}$ groups are present. Thus, the $+{I}$ effect is more in $\left({C _2} {H _5}\right) _{2} {NH}$ than in ${C _2} {H _5} {NH _2}$. Therefore, the electron density over the ${N}$-atom is more in $\left({C _2} {H _5}\right) _{2} {NH}$ than in ${C _2} {H _5} {NH _2}$. Hence, $\left({C _2} {H _5}\right) _{2} {NH}$ is more basic than ${C _2} {H_5} {NH_2}$.
Also, both ${C_6} {H_5} {NHCH_3}$ and ${C_6} {H_5} {NH_2}$ are less basic than $\left({C_2} {H_5}\right)_{2} {NH}$ and ${C_2} {H_5} {NH_2}$ due to the delocalization of the lone pair in the former two. Further, among ${C_6} {H_5} {NHCH_3}$ and ${C_6} {H_5} {NH_2}$, the former will be more basic due to the $+{I}$ effect of $-{CH_3}$ group. Hence, the order of increasing basicity of the given compounds is as follows:
${C_6} {H_5} {NH_2}<{C_6} {H_5} {NHCH_3}<{C_2} {H_5} {NH_2}<\left({C_2} {H_5}\right)_{2} {NH}$
We know that the higher the basic strength, the lower is the ${p} K_{b}$ values.
${C_6} {H_5} {NH_2}>{C_6} {H_5} {NHCH_3}>{C_2} {H_5} {NH_2}>\left({C_2} {H_5}\right)_{2} {NH}$
(ii) ${C _6} {H _5} {~N}\left({CH _3}\right) _{2}$ is more basic than ${C _6} {H _5} {NH _2}$ due to the presence of the $+{I}$ effect of two ${CH _3}$ groups in ${C _6} {H _5} {~N}\left({CH _3}\right) _{2}$. Further, ${CH _3} {NH _2}$ contains one $-{CH _3}$ group while $\left({C _2} {H _5}\right) _{2} {NH}$ contains two $-{C _2} {H _5}$ groups. Thus, $\left({C _2} {H _5}\right) _{2} {NH}$ is more basic than ${C _2} {H _5} {NH _2}$.
Now, ${C_6} {H_5} {~N}\left({CH_3}\right)_{2}$ is less basic than ${CH_3} {NH_2}$ because of the-R effect of $-{C_6} {H_5}$ group.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
${C _6} {H _5} {NH _2}<{C _6} {H _5} {~N}\left({CH _3}\right) _{2}<{CH _3} { NH _2}<\left({C _2} { H _5}\right) _{2} {NH}$
(iii)
(a)

In $p$-toluidine, the presence of electron-donating $-{CH_3}$ group increases the electron density on the ${N}$-atom. Thus, $p$-toluidine is more basic than aniline.
On the other hand, the presence of electron-withdrawing $-{NO_2}$ group decreases the electron density over the ${N}$-atom in $p$-nitroaniline. Thus, $p$-nitroaniline is less basic than aniline.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
$ p $-Nitroaniline $ < $ Aniline $ < p $-Toluidine
(b) ${C_6} {H_5} {NHCH_3}$ is more basic than ${C_6} {H_5} {NH_2}$ due to the presence of electron-donating - ${CH_3}$ group in ${C_6} {H_5} {NHCH_3}$.
Again, in ${C_6} {H_5} {NHCH_3},-{C_6} {H_5}$ group is directly attached to the ${N}$-atom. However, it is not so in ${C_6} {H_5} {CH_2} {NH_2}$. Thus, in ${C_6} {H_5} {NHCH_3}$, the - ${R}$ effect of $-{C_6} {H_5}$ group decreases the electron density over the ${N}$-atom. Therefore, ${C_6} {H_5} {CH_2} {NH_2}$ is more basic than ${C_6} {H_5} {NHCH_3}$.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
${C_6} {H_5} {NH_2}<{C_6} {H_5} {NHCH_3}<{C_6} {H_5} {CH_2} {NH_2}$.
(iv) In the gas phase, there is no solvation effect. As a result, the basic strength mainly depends upon the $+{I}$ effect. The higher the $+{I}$ effect, the stronger is the base. Also, the greater the number of alkyl groups, the higher is the $+{I}$ effect. Therefore, the given compounds can be arranged in the decreasing order of their basic strengths in the gas phase as follows:
$\left({C _2} {H _5}\right) _{3} {~N}>\left({C _2} {H _5}\right) _{2} {NH}>{C _2} {H _5} {NH _2}>{NH _3}$
(v) The boiling points of compounds depend on the extent of ${H}$-bonding present in that compound. The more extensive the H-bonding in the compound, the higher is the boiling point. $\left({CH _3}\right) _{2} {NH}$ contains only one ${H}$-atom whereas ${C _2} {H _5} {NH _2}$ contains two ${H}$-atoms. Then, ${C _2} {H _5} {NH _2}$ undergoes more extensive ${H}$-bonding than $\left({CH _3}\right) _{2} {NH}$. Hence, the boiling point of ${C _2} {H _5} {NH _2}$ is higher than that of $\left({CH _3}\right) _{2} {NH}$.
Further, ${O}$ is more electronegative than ${N}$. Thus, ${C_2} {H_5} {OH}$ forms stronger ${H}$-bonds than ${C_2} {H_5} {NH_2}$. As a result, the boiling point of ${C_2} {H_5} {OH}$ is higher than that of ${C_2} {H_5} {NH_2}$ and $\left({CH_3}\right)_{2} {NH}$.
Now, the given compounds can be arranged in the increasing order of their boiling points as follows:
$\left({CH_3}\right)_{2} {NH}<{C_2} {H_5} {NH_2}<{C_2} {H_5} {OH}$
(vi) The more extensive the H-bonding, the higher is the solubility. ${C _2} {H _5} {NH _2}$ contains two ${H}$ atoms whereas $\left({C _2} {H _5}\right) _{2} {NH}$ contains only one ${H}$-atom. Thus, ${C _2} {H _5} {NH _2}$ undergoes more extensive ${H}$-bonding than $\left({C _2} {H _5}\right) _{2} {NH}$. Hence, the solubility in water of ${C _2} {H _5} {NH _2}$ is more than that of $\left({C _2} {H _5}\right) _{2} {NH}$.
Further, the solubility of amines decreases with increase in the molecular mass. This is because the molecular mass of amines increases with an increase in the size of the hydrophobic part. The molecular mass of ${C_6} {H_5} {NH_2}$ is greater than that of ${C_2} {H_5} {NH_2}$ and $\left({C_2} {H_5}\right)_{2} {NH}$.
Hence, the increasing order of their solubility in water is as follows:
${C_6} {H_5} {NH_2}<\left({C_2} {H_5}\right)_{2} {NH}<{C_2} {H_5} {NH_2}$
13.5 How will you convert:
(i) Ethanoic acid into methanamine
(ii) Hexanenitrile into 1-aminopentane
(iii) Methanol to ethanoic acid
(iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine
(viii) Propanoic acid into ethanoic acid?
Show Answer
Answer
(i)

(ii)

(iii)

(iv)

(v)

(vi)

(vii)

(viii)

13.6 Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Show Answer
Answer
Benzenesulphonyl chloride $\left({C}_6 {H}_5 {SO}_2 {Cl}\right)$, which is also known as Hinsberg’s reagent, reacts with primary and secondary amines to form sulphonamides.
(a) The reaction of benzenesulphonyl chloride with primary amine yields N -ethylbenzenesulphonyl amide.

The hydrogen attached to nitrogen in sulphonamide is strongly acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is soluble in alkali.
(b) In the reaction with secondary amine, N,N-diethylbenzenesulphonamide is formed.

Since N, N-diethylbenzene sulphonamide does not contain any hydrogen atom attached to nitrogen atom, it is not acidic and hence insoluble in alkali.
(c) Tertiary amines do not react with benzenesulphonyl chloride. This property of amines reacting with benzenesulphonyl chloride in a different manner is used for the distinction of primary, secondary and tertiary amines and also for the separation of a mixture of amines. However, these days benzenesulphonyl chloride is replaced by $p$-toluenesulphonyl chloride.
13.7 Write short notes on the following:
(i) Carbylamine reaction
(ii) Diazotisation
(iii) Hofmann’s bromamide reaction
(iv) Coupling reaction
(v) Ammonolysis
(vi) Acetylation
(vii) Gabriel phthalimide synthesis.
Show Answer
Answer
(i) Carbylamine reaction
Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines which are foul smelling substances. Secondary and tertiary amines do not show this reaction. This reaction is known as carbylamine reaction or isocyanide test and is used as a test for primary amines.
$ \underset{\text{Primary amine}}{{R}-{NH_2}}+\underset{\text{Chloform}}{{CHCl_3}}+\underset{\substack{\text{Potassium}\\ \text{hyroxide}}}{3 {KOH}(\text { alc. })} \xrightarrow{\Delta} \underset{\text{Carbylamine}}{{R}-{NC}}+3 {KCl}+3 {H_2} {O} $
For example,
$ \underset{\text{Methanamine}}{{CH_3}-{NH_2}}+{CHCl_3}+3 {KOH}(\text {alc.}) \xrightarrow{\Delta} \underset{\substack{\text{Methyl carbylamine}\\ \text{or methyl isocyanide}}}{{CH_3}-{NC}}+3 {KCl}+3 {H_2} {O} $
(ii) Diazotisation
Aromatic primary amines react with nitrous acid (prepared in situ from ${NaNO_2}$ and a mineral acid such as ${HCl}$ ) at low temperatures (273-278 K) to form diazonium salts. This conversion of aromatic primary amines into diazonium salts is known as diazotization.
For example, on treatment with ${NaNO_2}$ and ${HCl}$ at $273 - 278 {~K}$, aniline produces benzenediazonium chloride, with ${NaCl}$ and ${H_2} {O}$ as by-products.

(iii) Hoffmann bromamide reaction
When an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide, a primary amine with one carbon atom less than the original amide is produced. This degradation reaction is known as Hoffmann bromamide reaction. This reaction involves the migration of an alkyl or aryl group from the carbonyl carbon atom of the amide to the nitrogen atom.

For example,

(iv) Coupling reaction
The azo products obtained have an extended conjugate system having both the aromatic rings joined through the $-{N}={N}$ - bond. These compounds are often coloured and are used as dyes. Benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position is coupled with the diazonium salt to form p-hydroxyazobenzene. This type of reaction is known as coupling reaction.

It can be observed that, the para-positions of phenol and aniline are coupled with the diazonium salt. This reaction proceeds through electrophilic substitution.
(v) Ammonolysis
When an alkyl or benzyl halide is allowed to react with an ethanolic solution of ammonia, it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino ( - ${NH_2}$ ) group. This process of cleavage of the carbon-halogen bond is known as ammonolysis.

The free amine can be obtained from the ammonium salt by treatment with a strong base :
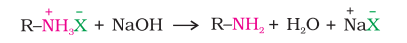
Ammonolysis has the disadvantage of yielding a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt. However, primary amine is obtained as a major product by taking large excess of ammonia.

(vi) Acetylation
Acetylation (or ethanoylation) is the process of introducing an acetyl group into a molecule.
Aliphatic and aromatic primary and secondary amines undergo acetylation reaction by nucleophilic substitution when treated with acid chlorides, anhydrides or esters. This reaction involves the replacement of the hydrogen atom of - ${NH_2} {Or}>{NH}$ group by the acetyl group, which in turn leads to the production of amides. To shift the equilibrium to the right hand side, the ${HCl}$ formed during the reaction is removed as soon as it is formed. This reaction is carried out in the presence of a base (such as pyridine) which is stronger than the amine.


When amines react with benzoyl chloride, the reaction is also known as benzoylation.
For example,

(vii) Gabriel phthalimide synthesis
Gabriel phthalimide synthesis is a very useful method for the preparation of aliphatic primary amines. It involves the treatment of phthalimide with ethanolic potassium hydroxide to form potassium salt of phthalimide. This salt is further heated with alkyl halide, followed by alkaline hydrolysis to yield the corresponding primary amine.

13.8 Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to $m$-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2,4,6-tribromofluorobenzene
(v) Benzyl chloride to 2-phenylethanamine
(vi) Chlorobenzene to $p$-chloroaniline
(vii) Aniline to $p$-bromoaniline
(viii) Benzamide to toluene
(ix) Aniline to benzyl alcohol.
Show Answer
Answer
(i)

(ii)

(iii)

(iv)

(v)

(vi)

(vii)

(viii)

(ix)

13.09 Give the structures of A, B and C in the following reactions:
(i) ${CH}_3 {CH}_2 {I} \xrightarrow{{NaCN}} {A} \xrightarrow[\text { Partial hydrolysis }]{{OH}^{-}} {B} \xrightarrow{{NaOH}+{Br}_2} {C}$
(ii) ${C}_6 {H}_5 {~N}_2 {Cl} \xrightarrow{{CuCN}} {A} \xrightarrow{{H}_2 {O} / {H}^{+}} {B} \xrightarrow[\Delta]{{NH}_3} {C}$
(iii) ${CH}_3 {CH}_2 {Br} \xrightarrow{{KCN}} {A} \xrightarrow{{LiAlH}_4} {~B} \xrightarrow[0^{\circ} {C}]{{HNO}_2} {C}$
(iv) ${C}_6 {H}_5 {NO}_2 \xrightarrow{{Fe} / {HCl}} {A} \xrightarrow[273 {~K}]{{NaNO}_2+{HCl}} {B} \xrightarrow[\Delta]{{H}_2 {O} / {H}^{+}} {C}$
(v) ${CH}_3 {COOH} \xrightarrow[\Delta]{{NH}_3} {~A} \xrightarrow{{NaOBr}} {B} \xrightarrow{{NaNO}_2 / {HCl}} {C}$
(vi) ${C}_6 {H}_5 {NO}_2 \xrightarrow{{Fe} / {HCl}} {A} \xrightarrow[273 {~K}]{{HNO}_2} {~B} \xrightarrow{{C}_6 {H}_5 {OH}} {C}$
Show Answer
Answer
(i) ${CH}_3 {CH}_2 {I} \xrightarrow{{NaCN}} {CH}_3 {CH}_2 {CN} \xrightarrow[\text { Partial hydrolysis }]{{OH}^{-}} {{CH}_3 {CH}_2CO{NH}_2} \xrightarrow{{NaOH}+{Br}_2} {{CH}_3 {CH}_2{NH}_2}$
(ii) ${C}_6 {H}_5 {~N}_2 {Cl} \xrightarrow{{CuCN}} {C_6H_5CN } \xrightarrow{{H}_2 {O} / {H}^{+}} {C_6H_5COOH} \xrightarrow[\Delta]{{NH}_3} {C_6H_5CONH_2}$
(iii) ${CH}_3 {CH}_2 {Br} \xrightarrow{{KCN}} {CH_3CH_2CN} \xrightarrow{{LiAlH}_4} {CH_3CH_2CH_2NH_2} \xrightarrow[0^{\circ} {C}]{{HNO}_2} {CH_3CH_2CH_2OH}$
(iv) ${C}_6 {H}_5 {NO}_2 \xrightarrow{{Fe} / {HCl}} {C_6H_5NH_2} \xrightarrow[273 {~K}]{{NaNO}_2+{HCl}} {C_6H_5N_2Cl} \xrightarrow[\Delta]{{H}_2 {O} / {H}^{+}} {C_6H_5OH}$
(v) ${CH}_3 {COOH} \xrightarrow[\Delta]{{NH}_3} {CH_3CONH_2} \xrightarrow{{NaOBr}} {CH_3NH_2} \xrightarrow{{NaNO}_2 / {HCl}} {CH_3OH}$
(vi) ${C}_6 {H}_5 {NO}_2 \xrightarrow{{Fe} / {HCl}} {C_6H_5NH_2} \xrightarrow[273 {~K}]{{HNO}_2} {C_6H_5N_2Cl} \xrightarrow{{C}_6 {H}_5 {OH}} {C_6H_5OH}$
13.10 An aromatic compound ’ ${A}$ ’ on treatment with aqueous ammonia and heating forms compound ’ ${B}$ ’ which on heating with ${Br_2}$ and ${KOH}$ forms a compound ’ ${C}$ ’ of molecular formula ${C_6} {H_7} {~N}$. Write the structures and IUPAC names of compounds ${A}, {B}$ and ${C}$.
Show Answer
Answer
It is given that compound ’ ${C}$ ’ having the molecular formula, ${C_6} {H_7} {~N}$ is formed by heating compound ‘B’ with ${Br_2}$ and ${KOH}$. This is a Hoffmann bromamide degradation reaction. Therefore, compound ’ ${B}$ ’ is an amide and compound ’ ${C}$ ’ is an amine. The only amine having the molecular formula, ${C_6} {H_7} {~N}$ is aniline, $\left({C_6} {H_5} {NH_2}\right)$.

Therefore, compound ‘B’ (from which ’ ${C}$ ’ is formed) must be benzamide, $\left({C_6} {H_5} {CONH_2}\right)$.

Further, benzamide is formed by heating compound ‘A’ with aqueous ammonia. Therefore, compound ‘A’ must be benzoic acid.

Benzoic acid
The given reactions can be explained with the help of the following equations:

13.11 Complete the following reactions:
(i) ${C_6} {H_5} {NH_2}+{CHCl_3}+$ alc. ${KOH} \rightarrow$
(ii) ${C_6} {H_5} {~N_2} {Cl}+{H_3} {PO_2}+{H_2} {O} \rightarrow$
(iii) ${C_6} {H_5} {NH_2}+{H_2} {SO_4}$ (conc.) $\rightarrow$
(iv) ${C_6} {H_5} {~N_2} {Cl}+{C_2} {H_5} {OH} \rightarrow$
(v) ${C_6} {H_5} {NH_2}+{Br_2}({aq}) \rightarrow$
(vi) ${C_6} {H_5} {NH_2}+\left({CH_3} {CO}\right)_{2} {O} \rightarrow$
(vii) ${C} _{6} {H} _{5} {~N} _{2} {Cl} \xrightarrow[\text { (ii) } {NaNO} _{2} / {Cu}, \Delta]{\left(\text { i) } {HBF} _{4}\right.}$
Show Answer
Answer
(i)
$ \begin{aligned} & {C}_6 {H}_5 {NH}_2+{CHCl}_3+3 {KOH} \rightarrow \underset{\text { Phenyl isocyanide }}{{C}_6 {H}_5 {NC}} +3 {KCl}+3 {H}_2 {O} \end{aligned} $
(ii) $\underset{\substack{\text{Benzenediazonium}\\ \text{chloride}}}{{C_6} {H_5} {~N_2} {Cl}}+{H_3} {PO_2}+{H_2} {O} \rightarrow \underset{\text{Benzene}}{{C_6} {H_6}}+{N_2}+{H_3} {PO_3}+{HCl}$
(iii)

(iv) $ \underset{\substack{\text{Benzenediazonium}\\ \text{chloride}}}{{C_6} {H_5} {N_2} {Cl}}+\underset{\text{Ethanol}}{{C_2} {H_5} {OH}} \rightarrow \underset{\text{Benzene}}{{C_6} {H_6}}+ \underset{\text{Ethanal}}{{CH_3} {CHO}}+{N_2}+{HCl} $
(v)

(vi)

(vii)

13.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Show Answer
Answer
Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine.

Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.

Hence, aromatic primary amines cannot be prepared by this process.
13.13 Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid.
Show Answer
Answer
(i) Aromatic amines react with nitrous acid (prepared in situ from ${NaNO_2}$ and a mineral acid such as ${HCl}$ ) at 273 - $278 {~K}$ to form stable aromatic diazonium salts i.e., ${NaCl}$ and ${H_2} {O}$.

(ii) Aliphatic primary amines react with nitrous acid (prepared in situ from ${NaNO_2}$ and a mineral acid such as ${HCl}$ ) to form unstable aliphatic diazonium salts, which further produce alcohol and ${HCl}$ with the evolution of ${N_2}$ gas.

13.14 Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
Show Answer
Answer
(i) Amines are less acidic than alcohols of comparable molecular masses. The reason is explained below:
- Amines lose a proton to form an amide ion. Alcohols lose a proton to form alkoxide ions.
- Formation of an amide ion: ${R}-{NH}_2 \longrightarrow {R}-{NH}^{-}+{H}^{+}$
- Formation of an alkoxide ion: ${R}-{OH} \longrightarrow {R}-{O}^{-}+{H}^{+}$
- Since oxygen is more electronegative than nitrogen, So, the alkoxide ion is more stable as compared to an amide ion. Hence, alcohol is more acidic than amines
(ii) In primary amines, nitrogen atoms have two hydrogen atoms which result in extensive intermolecular ${H}$ - bonding. In tertiary amines, nitrogen atoms do not have any hydrogen atoms and hydrogen bonding is not possible.
Hence, primary amines have a higher boiling point than tertiary amines.

Part (iii) : Aliphatic amines are stronger bases than aromatic amines due to following reasons: (a) The lone pair of electrons of the nitrogen atom of aromatic amines is involved in conjugation with the $\pi$-bond pairs of the ring as follows

(b) Anilinium ion obtained by accepting a proton is less stabilized by resonance. Due to the $-{R}$ effect of the benzene ring, the electrons on the ${N}$ atom are less available in the case of aromatic amines. Therefore, the electrons on the ${N}$-atom in aromatic amines cannot be donated easily.
We know that greater the number of resonating structures, greater is the stability. Thus you can infer that aniline (five resonating structures) is more stable than anilinium ion. Hence, the proton acceptability or the basic nature of aniline or other aromatic amines would be less than that of ammonia.
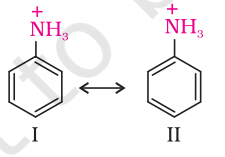
So, aniline is a weaker base than alkyl amines, in which $+I$ effect increases the electron density on the nitrogen atom.





