Unit 14 Biomolecules (Exercises)
Exercises
14.1 What are monosaccharides ?
Show Answer
Answer
Monosaccharides are carbohydrates which cannot be hydrolysed to smaller molecules. Their general formula is $\left({CH}_2 {O}\right)_n$ where $n=3-7$. These are of two types. Those which contain an aldehyde $(-{CHO})$ group are called aldoses and those which contain a keto ( ${C}={O}$ ) group are called ketoses. They are further classified as trioses, tetroses, pentoses, hexoses and heptoses according as they contain $3,4,5,6$ and 7 carbon atoms respectively. For example,

14.2 What are reducing sugars ?
Show Answer
Answer
Carbohydrates which reduce Fehling’s solution to red ppt. of ${Cu}_2 {O}$ or Tollens’ reagent to shining metallic Ag are called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except sucrose are reducing sugars. Thus, D-(+)-glucose, D-(+)-galactose, D-(-)-fructose, D-(+)-maltose and D-(+)-lactose are all reducing sugars.
14.3 Write two main functions of carbohydrates in plants.
Show Answer
Answer
(i) Structural material for plant cell walls. The polysaccharide cellulose acts as the chief structural material of the plant cell walls.
(ii) Reserve food material. The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and acts as the reserve food material for the tiny plant till it is capable of making its own food by photosynthesis.
14.4 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Show Answer
Answer
Monosaccharides : Ribose, 2-deoxyribose, galactose and fructose.
Disaccharides : Maltose and lactose.
14.5 What do you understand by the term glycosidic linkage ?
Show Answer
Answer
The ethereal or oxygen linkage through which two monosaccharides are joined together by the loss of a water molecule to form a molecule of disaccharide is called the glycosidic linkage. The glycosidic linkage in maltose molecule is shown below :

14.6 What is glycogen ? How is it different from starch ?
Show Answer
Answer
Starch is not a single compound but is a mixture of two components-a water soluble component called amylose $( 15-20 \% ) $ and water insoluble component called amylopectin $(80-85 \% )$. Amylose is a linear polymer of $\alpha$-D-glucose. But both glycogen and amylopectin are branched polymers of $\alpha$-Dglucose ; rather glycogen is more highly branched than amylopectin. Whereas amylopectin chains consist of $20-25$ glucose units, glycogen chains consist of $10-14$ glucose units.
14.7 What are the hydrolysis products of
(i) sucrose and
(ii) lactose ?
Show Answer
Answer
Both sucrose and lactose are disaccharides. Sucrose on hydrolysis gives one molecule each of glucose and fructose but lactose on hydrolysis gives one molecule each of glucose and galactose.

(ii) The hydrolysis of lactose gives $\beta$-D-galactose and $\beta$-D-glucose.

14.8 What is the basic structural difference between starch and cellulose ?
Show Answer
Answer
Starch consists of two components - amylose and amylopectin. Amylose is a long linear chain of $\alpha-D-(+)$-glucose units joined by C1-C4 glycosidic linkage ( $\alpha$-link).

Amylopectin is a branched-chain polymer of $\alpha$-D-glucose units, in which the chain is formed by C1-C4 glycosidic linkage and the branching occurs by C1-C6 glycosidic linkage.

On the other hand, cellulose is a straight-chain polysaccharide of $\beta$-D-glucose units joined by C1-C4 glycosidic linkage ($\beta$-link).

14.9 What happens when D-glucose is treated with the following reagents ?
(i) ${HI}$
(ii) $Bromine water$
(iii) ${HNO_3}$
Show Answer
Answer
(i) When D-glucose is heated with ${HI}$ for a long time, ${n}$-hexane is formed.

(ii) When D-glucose is treated with ${Br_2}$ water, D- gluconic acid is produced.

(iii) On being treated with ${HNO_3}$, D-glucose get oxidised to give saccharic acid.

14.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Show Answer
Answer
(1) Aldehydes give 2, 4-DNP test, Schiff’s test, and react with ${NaHSO_4}$ to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free - ${CHO}$ group is absent from glucose.
(3) Glucose exists in two crystalline forms - $\alpha$ and $\beta$. The $\alpha$-form (m.p. $=419 {~K}$ ) crystallises from a concentrated solution of glucose at $303 {~K}$ and the $\beta$-form (m.p $=423 {~K}$ ) crystallises from a hot and saturated aqueous solution at $371 {~K}$. This behaviour cannot be explained by the open chain structure of glucose.
14.11 What are essential and non-essential amino acids ? Give two examples of each type.
Show Answer
Answer
$\alpha$-Amino acids which are needed for health and growth of human beings but are not synthesized by the human body are called essential amino acids. For example, valine, leucine, phenylalanine, etc. On the other hand, $\alpha$-amino acids which are needed for health and growth of human beings and are synthesized by the human body are called non-essential amino acids. For example, glycine, alanine, aspartic acid, etc.
14.12 Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Show Answer
Answer
(i) Peptide linkage:
The amide formed between $-{COOH}$ group of one molecule of an amino acid and $-{NH_2}$ group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.

(ii) Primary structure:
The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
(iii) Denaturation:
In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in ${pH}$, its ${H}$-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation. During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered.
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
14.13 What are the common types of secondary structure of proteins ?
Show Answer
Answer
There are two common types of secondary structure of proteins:
(i) $\propto$-helix structure
(ii) $\beta$-pleated sheet structure
$\alpha$ - Helix structure:
In this structure, the $-{NH}$ group of an amino acid residue forms ${H}$-bond with the $ \rangle {C=O}$ group of the adjacent turn of the right-handed screw ( $\alpha$-helix).
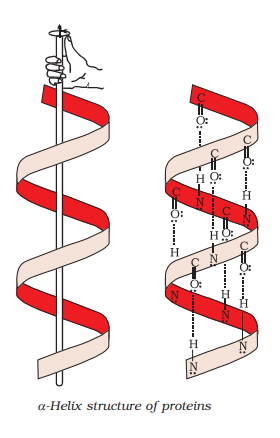
$\beta$-pleated sheet structure:
This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.

14.14 What type of bonding helps in stabilising the $\alpha$-helix structure of proteins ?
Show Answer
Answer
The H-bonds formed between the - ${NH}$ group of each amino acid residue and the $\rangle {{C}}={O}$ group of the adjacent turns of the $\alpha$-helix help in stabilising the helix.
14.15 Differentiate between globular and fibrous proteins.
Show Answer
Answer
| Property | Globular Proteins | Fibrous Proteins |
|---|---|---|
| Shape | Globular proteins have almost spheroidal shape due to folding of the polypeptide chain. | Polypeptide chains of fibrous proteins consist of thread-like molecules which tend to lie side by side to form fibres. |
| Solubility | Globular proteins are soluble in water. | Fibrous proteins are insoluble in water. |
| Sensitivity to Temperature and pH | Globular proteins are sensitive to small changes of temperature and pH. Therefore, they undergo denaturation on heating or on treatment with acids/bases. | Fibrous proteins are stable to moderate changes of temperature and pH. |
| Biological Activity | Globular proteins possess biological activity. That is why they act as enzymes (maltase, invertase, etc.), hormones (insulin), antibodies (gamma globulins), transport agents (haemoglobin), etc. | Fibrous proteins do not have any biological activity but serve as chief structural material of animal tissues. For example, keratin in skin, hair, nails, and wool, collagen in tendons, fibroin in silk, and myosin in muscles. |
14.16 How do you explain the amphoteric behaviour of amino acids ?
Show Answer
Answer
Amino acids contain an acidic (carboxyl group) and a basic (amino) group within the same molecule. In aqueous solution, they neutralize each other. The carboxyl group loses a proton while the amino group accepts it. As a result, a dipolar or zwitterion is formed.

In zwitterionic form, $\alpha$-amino acids show amphoteric behaviour as they react with both acids and bases. In the acidic medium, $\mathrm{COO}^{-}$ ion of the zwitterion accepts a proton to form the cation while in the basic medium, $\stackrel{+}{\mathrm{N}} \mathrm{H}_3$ ion loses a proton to form the anion .Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base.

Thus, amino acids show amphoteric behaviour.
14.17 What are enzymes ?
Show Answer
Answer
Enzymes are biological catalysts. Each biological system requires a different enzyme. Thus, as compared to conventional catalysts, enzymes are very specific and efficient in their action. They are required in only small quantity and work at optimum temperature $(310 \mathrm{~K})$ and $\mathrm{pH}(7-4)$ under one atmospheric pressure. Chemically, they are globular proteins. However, some enzymes are also associated with some non-protein component called the cofactor for their activity.
Cofactors are of two types :
(a) Inorganic ions such as $\mathrm{Zn}^{2+}, \mathrm{Mg}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}, \mathrm{Cu}^{2+}, \mathrm{Co}^{2+}$, etc.
(b) Organic molecules : These are also of two types :
(i) Coenzymes : These are usually derived from , vitamins such as thaimine, riboflavin, niacin, etc. They are loosely held to the protein and can be easily separated by dialysis.
(ii) Prosthetic group : These are also derived from vitamins such as biotin but are tightly held to the protein molecule by covalent bonds. They can be separated only by careful hydrolysis.
14.18 What is the effect of denaturation on the structure of proteins ?
Show Answer
Answer
During denaturation, $2^{\circ}$ and $3^{\circ}$ structures of proteins are destroyed but $1^{\circ}$ structure remains intact. As a result of denaturation, the globular proteins (soluble in $\mathrm{H}_2 \mathrm{O}$ ) are converted into fibrous proteins (insoluble in $\mathrm{H}_2 \mathrm{O}$ ) and their biological activity is lost. For example, boiled egg which contains coagulated proteins cannot be hatched to produce chickens.
14.19 How are vitamins classified ? Name the vitamin responsible for the coagulation of blood.
Show Answer
Answer
Vitamins are classified into two groups depending upon their solubility in water or fat.
(i) Water soluble vitamins : These include vitamin B - complex $\left(\mathrm{B}_1, \mathrm{~B}_2, \mathrm{~B}_3\right.$, i.e., nicotinic acid, $\mathrm{B}_5$, i.e., pantothenic acid, $\mathrm{B}6, \mathrm{~B}{12}$ and folic acid) and vitamin C .
(ii) Fat soluble vitamins : These include vitamins A, D, E and K. They are stored in liver and adipose (fat storing tissues). However, biotin, i.e., vitamin H is neither soluble in water nor in fat. Vitamin K is responsible for coagulation of blood.
14.20 Why are vitamin A and vitamin C essential to us ? Give their important sources.
Show Answer
Answer
Vitamin A is essential to us because its deficiency causes xerophthalmia (hardening of cornea of eye) and night blindness.
Sources: Fish liver oil, carrots, butter and milk.
Vitamin C is essential to us because its deficiency causes scurvy (bleeding gums) and pyorrhea (loosening and bleeding of teeth).
Sources : Citrous fruits ; amla, green leafy vegetables.
14.21 What are nucleic acids ? Mention their two important functions.
Show Answer
Answer
Nucleic acids are biomolecules which are found in the nuclei of all living cells in form of nucleoproteins or chromosomes (proteins containing nucleic acids as the prosthetic group).
Nucleic acids are of two types : deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The two main functions of nucleic acids are :
(i) DNA is responsible for transmission of hereditary effects from one generation to another. This is due to the unique property of replication during cell division as a result of which two identical DNA strands are transferred to the daughter cells.
(ii) DNA and RNA are responsible for synthesis of all proteins needed for the growth and maintenance of our body. Actually the proteins are synthesized by various RNA molecules ( $r$-RNA, $m$-RNA and $t-$ RNA ) in the cell but the message for the synthesis of a particular protein is given by DNA molecules.
14.22 What is the difference between a nucleoside and a nucleotide ?
Show Answer
Answer
A nucleoside is formed when 1-position of a pyrimidine (cytosine, thymine or uracil) or 9-position of a purine (guanine or adenine) base is attached to C -1 of sugar (ribose or deoxyribose) by a $\beta$-linkage. Thus, in general, nucleosides may be represented as : Sugar-Base.
A nucleotide contains all the three basic components of nucleic acids, i.e., a phosphoric acid group, a pentose sugar and a nitrogenous base. These are obtained by esterification of $\mathrm{C}_5{ }^{\prime}-\mathrm{OH}$ group of the pentose sugar by phosphoric acid.

14.23 The two strands in DNA are not identical but are complementary. Explain.
Show Answer
Answer
The two strands in DNA molecule are held together by hydrogen bonds between purine base of one strand and pyrimidine base of the other and vice versa. Because of different sizes and geometries of the bases, the only possible pairing in DNA are G (guanine) and C (cytosine) through three H-bonds, i.e., $(\mathrm{C} \equiv \mathrm{G})$ and between A (adenine) and T (thymine) through two H-bonds (i.e, $\mathrm{A}=\mathrm{T}$ ) . Due to this base-pairing principle, the sequence of bases in one strand automatically fixes the sequence of bases in the other strand. Thus, the two strands are complementary and not identical.
14.24 Write the important structural and functional differences between DNA and RNA.
Show Answer
Answer
The structural differences between DNA and RNA are as follows:
| DNA | RNA | ||
|---|---|---|---|
| 1. | The sugar moiety in DNA molecules is $\beta$-D-2 deoxyribose. | 1. | The sugar moiety in RNA molecules is $\beta$-D-ribose. |
| 2. | DNA contains thymine (T). It does not contain uracil (U). | 2. | RNA contains uracil (U). It does not contain thymine (T). |
| 3. | The helical structure of DNA is double-stranded. | 3. | The helical structure of RNA is single- stranded. |
The functional differences between DNA and RNA are as follows:
| DNA | RNA | ||
|---|---|---|---|
| 1 | DNA is the chemical basis of heredity. | 1 | RNA is not responsible for heredity. |
| 2 | DNA molecules do not synthesise proteins, but transfer coded | 2 | Proteins are synthesised by RNA |
14.25 What are the different types of RNA found in the cell ?
Show Answer
Answer
(i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii) Transfer RNA (t-RNA)





