Chemical Bonding And Molecular Structure Ques 39
39. In $PO_4{ }^{3-}$ ion, the formal charge on each oxygen atom and $P-O$ bond order respectively are
(a) $-0.75,0.6$
(b) $-0.75,1.0$
(c) $-0.75,1.25$
(d) $-3,1.25$
[1998]
Show Answer
Solution:
- (c) Bond order between $P-O$
$=\frac{\text{ no. of bonds in all possible direction }}{\text{ total no. of resonating structures }}=\frac{5}{4}=1.25$
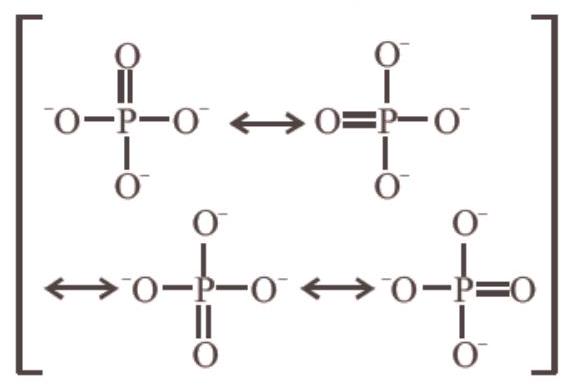
or Formal charge on oxygen $=-\frac{3}{4}=-0.75$





