The D And F Block Elements Ques 17
17. The aqueous solution containing which one of the following ions will be colourless? (Atomic number: $Sc=21, Fe=26, Ti=22, Mn$ $=25$ )
[2005]
(a) $Sc^{3+}$
(b) $Fe^{2+}$
(c) $Ti^{3+}$
(d) $Mn^{2+}$
Show Answer
Solution:
(a) $Sc^{3+} \to 3 d^{0} 4 s^{0}$
$Fe^{2+} \to 3 d^{6} 4 s^{0}$
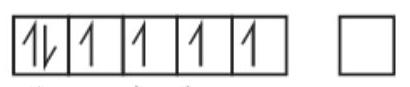
4 unpaired $e^{-}$
$ Ti^{3+} \to 3 d^{1} 4 s^{0} $
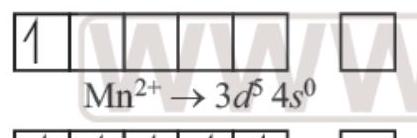
1 unpaired $e^{-}$
\begin{tabular}{|l|l|l|l|l|} \hline 1 & 1 & 1 & 1 & 1 \end{tabular}$\quad \square 5$ unpaired $e^{-}$
In $Sc^{3+}$ there is no unpaired electron. So the aqueous solution of $Sc^{3+}$ will be colourless.





