Qualitative Analysis - Result Question 46
49.
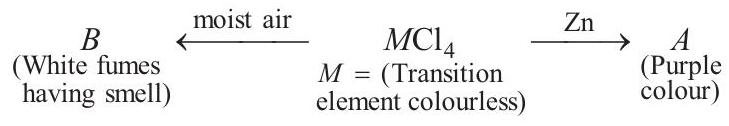
Identify the metal $M$ and hence $M Cl _4$. Explain the difference in colours of $M Cl _4$ and $A$.
(2005,4 M)
Show Answer
Solution:
$\underset{\text { Colourless }}{MCl _4} \stackrel{Zn}{\longrightarrow}$ Purple coloured compound $(A)$
$\underset{\text { Transition metal }}{M} \xrightarrow[\text { Air }]{\text { Moist }} B \text { (white fumes) }$
$\Rightarrow \quad M=Ti, \quad A=\left[Ti\left(H _2 O\right) _6\right]^{3+} ; \quad B=TiO _2$
Ti (IV) contains no $d$-electron, while $d$ - $d$ transition of single electron of $Ti$ (III) will cause colour change.





