Transition and InnerTransition Elements - Result Question 63
63.
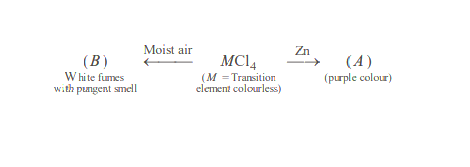
Identify the metal $M$ and hence $\mathrm{MCl}_4$. Explain the difference in colours of $\mathrm{MCl}_4$ and $A$.
(2005)
Show Answer
Solution:
$A=\left[\mathrm{Ti}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$ and $M=\mathrm{Ti}, B=\mathrm{TiO}_2, \mathrm{Ti}(\mathrm{IV})$ has no electron in $3 d$-orbital, no $d-d$ transition is possible, therefore $M \mathrm{Cl}_4$ is colourless. In $A$, there is one electron in $3 d$-orbital and its $d-d$ transition is responsible for colour.





